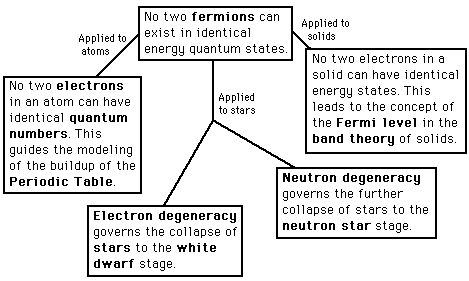
Pauli Exclusion Principle
No two electrons in an atom can have identical quantum numbers. This is an example of a general principle which applies not only to electrons but also to other particles of half-integer spin (fermions). It does not apply to particles of integer spin (bosons).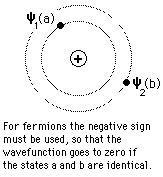 |
The nature of the Pauli exclusion principle can be illustrated by supposing that electrons 1 and 2 are in states a and b respectively. The wavefunction for the two electron system would be 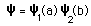 but this wavefunction is unacceptable because the electrons are identical. To account for this we must use a linear combination 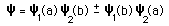 The Pauli exclusion principle is part of one of our most basic observations of nature: particles of half-integer spin must have antisymmetric wavefunctions, and particles of integer spin must have symmetric wavefunctions. |
Applications
| HyperPhysics***** Quantum Physics | R Nave |